Warning and Crack Down on Stem Cell Clinics in U.S. and Canada

U.S. FDA Issues Warning to Stem Cell Clinics
The U.S. Food and Drug Administration (FDA) has issued warning to U.S. Stem Cell clinics and biotech companies. FDA wants to make sure deceitful players do not take advantage of desperate patients by claiming they have cure for diseases without any proof that they work. “Purporting they have treatment for serious diseases without FDA approval is a significant deviations from current good manufacturing practice requirements,” FDA claims.
FDA just seized a San Diego stem cell company using Smallpox vaccine in risky cancer therapy. The agency is saying that it is critical to shut down “unscrupulous” marketing of regenerative medicine products (stem cell, gene therapies and immunotherapies).
Stem cells can be obtained from bone marrow, blood or fat. Only few stem cell products are approved by Food and Drug Administration. The companies FDA cited from using stem cell extracted from fat taken from patient’s belly to reinject to patient’s body.
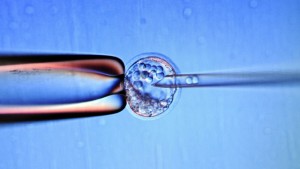 There are exciting and promising studies and research showing stem cells treat macular degeneration, Multiple Sclerosis (MS), diabetes and other fatal diseases. However, some of these treatments could put patients at risk. Patients may suffer harm or even death from unproven or unsafe treatments. For example, one stem cell treatment stabilizes macular degeneration and others blinds 3 patients.
There are exciting and promising studies and research showing stem cells treat macular degeneration, Multiple Sclerosis (MS), diabetes and other fatal diseases. However, some of these treatments could put patients at risk. Patients may suffer harm or even death from unproven or unsafe treatments. For example, one stem cell treatment stabilizes macular degeneration and others blinds 3 patients.
Stem cell therapy is like other medicine needs the FDA approval. Whether FDA start regulating more stem cell therapies is yet to be seen. Incidentally, FDA just cleared the first US gene therapy for cancer treatment (Leukemia).
Health Canada Looking into Stem Cell Clinics

Stem Cell Basics
All of 200 or more type of cells in human body is made of a single cell (Zygote) that forms when an egg is fertilized by an sperm. Zygote divides over and over to a form hollow ball of cells (blastocyst) which gives rise to different cells, such as the umbilical cord and the placenta. Each human cell has its own size and structure, and consists of membrane, nucleus (DNA), the liquid outside the nucleus (Cytoplasm).
Stem Cells are undifferentiated cells capable of self-renew and differentiate into different cell types and tissues. They can become specialized cells, divide continuously, and have immune potential to treat a wide range of medical problems (Regenerative Medicine). They are classified into:

- Unipotent
- Oligopotent
- Multipotent (several)
- Pluripotent (many) – True stem cells
- Totipotent (whole)
Stem cells are characterized into:
- Self-renw – They can copy themselves.
- Differentiate – They can develop into specialized cells.
Stem cells, the foundation of human development, are formed in different times in our lives.
- Embryonic Stem Cells (ES Cells) are formed and exists only at the very early stage from embryo (blastocyst) when is few days old. Embryonic stem cells are pluripotent, they can generate all different types of cells.
- Adult or Somatic (Tissue-Specific) Stem Cells that are formed during fetal development and stays in our bodies. Adult stem cells are mutipotent, they can generate multiple, organ-specific cell types.